The various immunoglobulin isotypes and classes have been mentioned briefly already.Each class is distinguished by unique amino acid sequences in heavy-chain constant region that confer class-specific structural and functional properties .In this section,the structure and effector functions of each class are described in more detail.
Immunoglobulin G (IgG):
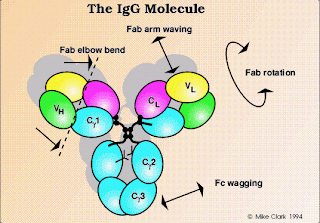
IgG,the most abundant class in serum,constitutes about 80% of the total serum immunoglobulin.The IgG molecule consists of two heavy chains and two light chains.There are four human IgG subclasses,distinguished by differences in heavy-chain sequence and numbered according to their decreasing average serum concentrations:IgG1,IgG2,IgG3,and IgG4.
The amino acid sequence that distinguish the four IgG subclasses are encoded by different germ-line genes,whose DNA sequences are 90%-95% homologous.The structural characteristics that distinguish these subclasses from one another are the size of the hinge region and the number and position of the interchain disulfide bonds between the heavy chains.The subtle amino acid differences between subclasses of IgG affect the biological activity of the molecule:
* IgG1,IgG3,and IgG4 readily cross the placenta and play an important role in protecting the developing fetus.
* IgG3 is the most effective complement at all.
* IgG1 and IgG3 bind with high affinity to Fc receptors on phagocytic cells and thus mediate opsonization.IgG4 has an intermediate affinity for Fc receptors,and IgG2 has an extremely low affinity.
Immunoglobulin G (IgG):
IgG,the most abundant class in serum,constitutes about 80% of the total serum immunoglobulin.The IgG molecule consists of two heavy chains and two light chains.There are four human IgG subclasses,distinguished by differences in heavy-chain sequence and numbered according to their decreasing average serum concentrations:IgG1,IgG2,IgG3,and IgG4.
The amino acid sequence that distinguish the four IgG subclasses are encoded by different germ-line genes,whose DNA sequences are 90%-95% homologous.The structural characteristics that distinguish these subclasses from one another are the size of the hinge region and the number and position of the interchain disulfide bonds between the heavy chains.The subtle amino acid differences between subclasses of IgG affect the biological activity of the molecule:
* IgG1,IgG3,and IgG4 readily cross the placenta and play an important role in protecting the developing fetus.
* IgG3 is the most effective complement at all.
* IgG1 and IgG3 bind with high affinity to Fc receptors on phagocytic cells and thus mediate opsonization.IgG4 has an intermediate affinity for Fc receptors,and IgG2 has an extremely low affinity.

0 comments