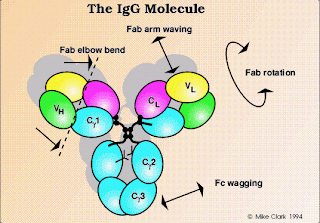
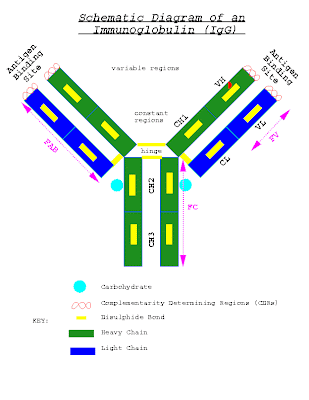
The factors that promotes Oxygen binding as well as Oxygen dissociation with respect to Hb are as follows:
1. Hb and partial pressure of Oxygen:
The most important factor that determine how much Oxygen combines with Hb is partialpressure of Oxygen.When the partial pressure is high i.e., in the pulmonary blood capillaries where partial pressure of Oxygen is low hold as much Oxygen and so Oxygen is released to the tissue cells.The relationship between saturation of Hb with respect to partial pressure can be illustrated by Oxygen-Hydrogen dissociative curve.
2. Hb and Acidity(Hb+pH):
In an acidic environment,Hb affinity for Oxygen is low as a result Oxygen spilts more easily from Hb (Bohr effect).When hydrogen ions bind to certain AAs in the polypeptide chain of Hb .They alter its structure and thereby decrease Hb's Oxygen carrying capacity.Thus a low pH (acidic medium)will always drive Oxygen away from Hb making available for the tissue cells.
3. Hb and BPG ( 2,3-Bisphosphoglycerate):
BPG is found in RBCs and is an important regulation og Hb function.It decreases affinity of Hb for Oxygen and thus helps to release Oxygen from Hb. BPG is formed in RBCs when they breakdown glucose for energy by the process called glycolysis.when BPG combines with Hb,Hb binds to Oxygen less tightly.The greater the level of BPG,the more will be the Oxygen released from Hb.
4. Hb and partial carbondioxide:
Carbondioxide can also bind to the Hb and the effect is similar like hydrogen ions.In the tissue bloodcapillary more acidic,this is because carbondioxide is temporarily converted into carbonic acid.This conversion is catalysed by the enzyme carbonic anhydrase,while formed in the RBCs.Carbonic acid being a weak acid dissociates into bicarbonate and hydrogen ions.As the hydrogen concentration increases,the pH decreases.As a result the blood becomes more acidic .In an acidic environment,Oxygen will spilt from Hb.
5. Temperature:
As the temperature increases the amount of Oxygen released from Hb also increases.Heat energy which is a by product of metabolic reaction of all the cells results in active cells,liberating more heat and requiring more Oxygen.The more active the cell,the more metabolic will be the state.In other words,more Carbondioxide will be released and more acidic will be the environment.the acid and the heat thus promote release of Oxygen from Hb.
The most important factor that determine how much Oxygen combines with Hb is partialpressure of Oxygen.When the partial pressure is high i.e., in the pulmonary blood capillaries where partial pressure of Oxygen is low hold as much Oxygen and so Oxygen is released to the tissue cells.The relationship between saturation of Hb with respect to partial pressure can be illustrated by Oxygen-Hydrogen dissociative curve.
2. Hb and Acidity(Hb+pH):
In an acidic environment,Hb affinity for Oxygen is low as a result Oxygen spilts more easily from Hb (Bohr effect).When hydrogen ions bind to certain AAs in the polypeptide chain of Hb .They alter its structure and thereby decrease Hb's Oxygen carrying capacity.Thus a low pH (acidic medium)will always drive Oxygen away from Hb making available for the tissue cells.
3. Hb and BPG ( 2,3-Bisphosphoglycerate):
BPG is found in RBCs and is an important regulation og Hb function.It decreases affinity of Hb for Oxygen and thus helps to release Oxygen from Hb. BPG is formed in RBCs when they breakdown glucose for energy by the process called glycolysis.when BPG combines with Hb,Hb binds to Oxygen less tightly.The greater the level of BPG,the more will be the Oxygen released from Hb.
4. Hb and partial carbondioxide:
Carbondioxide can also bind to the Hb and the effect is similar like hydrogen ions.In the tissue bloodcapillary more acidic,this is because carbondioxide is temporarily converted into carbonic acid.This conversion is catalysed by the enzyme carbonic anhydrase,while formed in the RBCs.Carbonic acid being a weak acid dissociates into bicarbonate and hydrogen ions.As the hydrogen concentration increases,the pH decreases.As a result the blood becomes more acidic .In an acidic environment,Oxygen will spilt from Hb.
5. Temperature:
As the temperature increases the amount of Oxygen released from Hb also increases.Heat energy which is a by product of metabolic reaction of all the cells results in active cells,liberating more heat and requiring more Oxygen.The more active the cell,the more metabolic will be the state.In other words,more Carbondioxide will be released and more acidic will be the environment.the acid and the heat thus promote release of Oxygen from Hb.