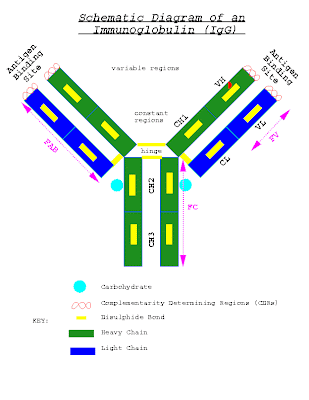
The structure of the immunoglobulin molecule is determined by the primary,secondary,tertiary, and quaternary organization of the protein.
The primary structure,the amino acid sequence,accounts for the variable and constant regions of the heavy and light chains.The secondary structure is formed by folding of the extended polypeptide chain back and forth upon itself into an antiparallel beta pleated sheet.
The chains are then folded into tertiary structure of compact globular domains by continuations of the polypeptide chain that lie outside the beta pleated.Finally,the globular domains of adjacent heavy and light polypeptide chains interact in the quaternary structure,forming functional domains that enable the molecule to specifically bind antigen and,at the same time,perform a number of biological effector functions.
The primary structure,the amino acid sequence,accounts for the variable and constant regions of the heavy and light chains.The secondary structure is formed by folding of the extended polypeptide chain back and forth upon itself into an antiparallel beta pleated sheet.
The chains are then folded into tertiary structure of compact globular domains by continuations of the polypeptide chain that lie outside the beta pleated.Finally,the globular domains of adjacent heavy and light polypeptide chains interact in the quaternary structure,forming functional domains that enable the molecule to specifically bind antigen and,at the same time,perform a number of biological effector functions.

0 comments